Research
The goal of our team is to facilitate in vitro development of large, complex tissues with an inherent vascular bed capable of rapid integration with the host. Concurrent growth of tissue structures with vasculature is necessary for nutrient delivery and support of differentiation. Thus, there is a need to understand and characterize neovascularization in vitro. Under appropriate three dimensional culture conditions, endothelial cells are capable of forming tubular networks that are further stabilized by the presence of mural cells. While these vascular structures are capable of integration with a host upon implantation, they are prone to failure and regression. The introduction of a controlled 3D environment – where nutrients, growth factors, oxygen and transport processes are delivered as needed – contributes to the formation of a stable, hierarchical vascular network. Our group is developing novel techniques, algorithms and methodologies for this purpose.
CELLULAR RESPONSE TO FLOW
It is widely accepted that cells respond to both chemical and physical stimuli. While much has been learned about chemical responses in the form of growth factors and signaling, less is understood about cellular responses to gravitational forces, mechanical stretch and shear stress. The endothelium, in particular is constantly subjected to mechanical forces and high shear stress associated with the flow of blood. It is our goal to develop experimental strategies that clarify the effect of flow in the organization of vascular patterns, differentiation of vascular phenotypes (arterial vs. venular) and integration of these vessels within the context of complex tissues.
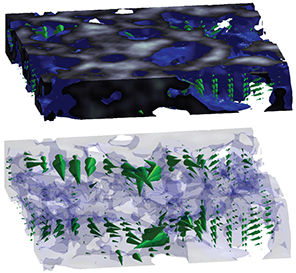
FLOW SIMULATIONS
Computational fluid dynamics (CFD) is used to predict the effect of macro- and micro-scale scaffold design and flow parameters (i.e. porosity, channel topology, inlet pressure, inlet distribution) on shear stress and flow field distribution. The flow conditions are believed to influence the proliferation, migration and morphogenesis of cells seeded within the scaffolds. The CFD predictions are used to find optimal designs and configurations that are subsequently applied to fabricate 3D polymer scaffolds. As a first approach, 2D flow simulations are used as an approximation to reduce computational time and complexity. However, more complex experimental situations will require 3D flow simulations, which are computationally extensive. The computational time required by these simulations can be in the order of days. Parallel processing techniques, along with efficient software algorithms, are used to boost the speed of 3D simulations. The CFD methods applied are based on the Lattice Boltzmann Method (LBM), which is known for its speed and efficiency due to the statistical nature of its equations that model fluid dynamics.
DESIGN OF BIODEGRADABLE POLYMER SCAFFOLDS
Porous scaffolds are fabricated by solvent casting of polycaprolactone (PCL) with a porogen phase. To achieve patterned scaffolds, the polymer/porogen solution is cast in a mold with imprinted channel features. A high porogen to polymer ratio is used in order to achieve high porosity and interconnectivity of the pores post leaching. The resulting porosity is ~90% with open pores on the surface of the scaffold. To calculate the interior porosity, X-ray microtomography and scanning electron microscopy is used to characterize the scaffold. The choice of PCL as the scaffolding material allows for a biocompatible material to form a porous network with mechanical and chemical stability to support cell growth and organization over the time duration of flow experiments. The scaffolds desired are macroporous with interconnected pores, such that fluid can flow and cells migrate within the scaffold.
3D HYDROGEL MATRICES
Natural extracellular matrix materials are used in combination to form three-dimensional gels and encapsulate primary cells. The components of collagen and fibrin are varied to find optimal mechanical properties and achieve cellular homeostasis. Rheological measurements are made on the hydrogel mixtures to correlate with morphological features as determined by scanning electron microscopy. The effect of matrix porosity and fibril distribution on fluid flow will be determined. Flow experiments on endothelial cells encapsulated in the hydrogel matrices will be performed to ultimately investigate the cellular response to flow in 3D.
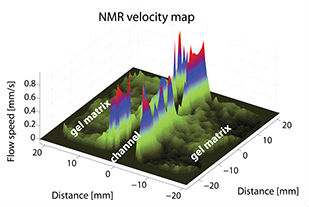
FLOW MAPPING
Methods are being devised to correlate flows with morphogenesis. We have developed a reliable technique based on nuclear magnetic resonance (NMR) microimaging, which is capable of noninvasively probing perfusion flows inside porous scaffolds. This NMR velocimetry technique enables us to obtain 3D flow maps in real time and study cell behavior as a function of applied flow conditions. We are working to achieve high spatial resolution to map flows from small enough volumes (voxels) where it is not much larger than a cell or cluster of cells. We have demonstrated that voxels with 80µm in-plane by 0.5mm thick sizes are possible.
Back to Top
